Our past conferences have been packed full of valuable information and outstanding networking opportunities, and we know this year will continue the tradition. Welcome to our 4th Global IDMP Working Group (GIDWG) Stakeholder Meeting that is now preliminary planned for third week of September 2024 and will be hosted by Brazilian Health Regulatory Agency (Agência Nacional de Vigilância Sanitária, Anvisa) in Brazil.
Dear All!
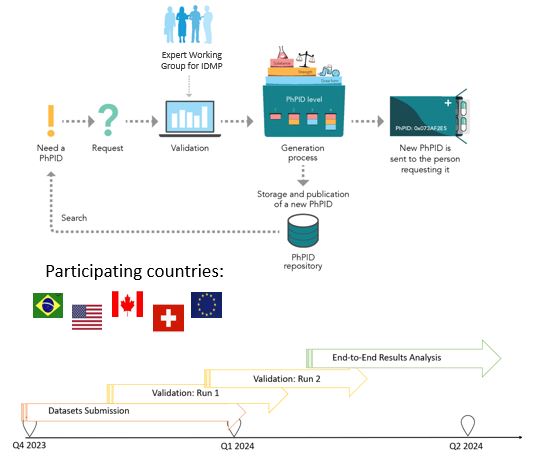
Thank You so much for joining the 4th GIDWG Technical and Stakeholder Meeting!
It has not only been a highly productive meeting but also a fantastic opportunity to come together and advance our shared vision of establishing a framework for truly global IDMP implementation.
Please find below presentations materials: