Testing of use cases for PhPID operating model
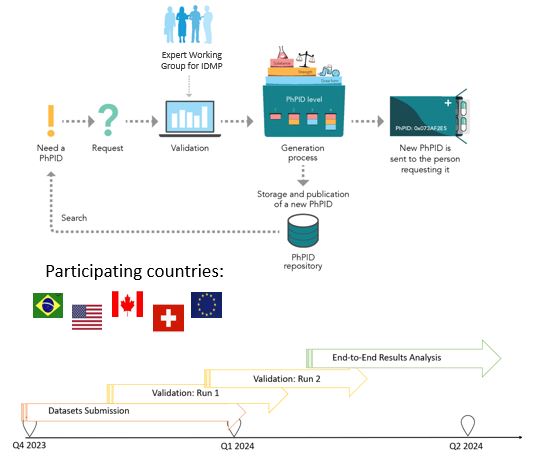
PURPOSE:
Testing framework, including business rules, best practices, software and operating model, for the global IDMP implementation and maintenance of global identifiers for marketed products.
SCOPE:
•Validate and generate PhPIDs for medicinal products based on GIDWG/EWG Business Rules
•EDQM + non-EDQM countries
•Similar products from different countries
•Larger batches & smaller data sets for regulators
•Testing of Pharmacovigilance, Drug Shortages and Cross-border Healthcare use cases
STATUS: ongoing
