Our past conferences have been packed full of valuable information and outstanding networking opportunities, and we know this year will continue the tradition. Welcome to our 4th Global IDMP Working Group (GIDWG) Stakeholder Meeting that is now preliminary planned for third week of September 2024 and will be hosted by Brazilian Health Regulatory Agency (Agência Nacional de Vigilância Sanitária, Anvisa) in Brazil.
News & Updates
End-to-End Demonstration Q4 2023 – Q2 2024
Testing of use cases for PhPID operating model
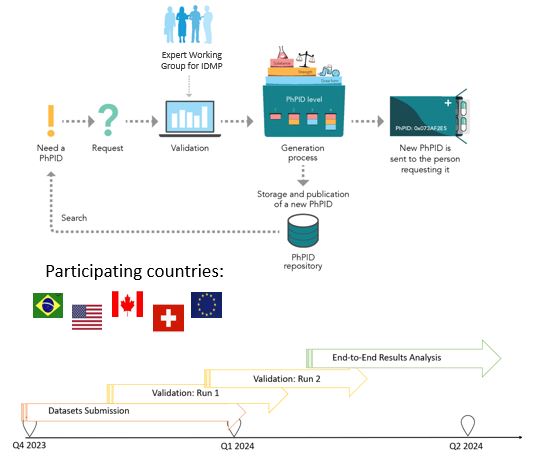
PURPOSE:
Testing framework, including business rules, best practices, software and operating model, for the global IDMP implementation and maintenance of global identifiers for marketed products.
SCOPE:
•Validate and generate PhPIDs for medicinal products based on GIDWG/EWG Business Rules
•EDQM + non-EDQM countries
•Similar products from different countries
•Larger batches & smaller data sets for regulators
•Testing of Pharmacovigilance, Drug Shortages and Cross-border Healthcare use cases
STATUS: ongoing
Podcast explains IDMP standards
The Identification of Medicinal Products (IDMP) standards promise to harmonise how pharmaceutical products and substances are described around the world. But how will that benefit patients and who will make sure the standards are properly implemented? Uppsala Monitoring Centre’s Malin Fladvad and Olle Lagerlund join the Drug Safety Matters podcast to discuss the advantages and challenges of this global standardisation effort.
