This document provides high-level guidance and recommendations from the Global IDMP Working Group (GIDWG) on how to assign Pharmaceutical Product Identifiers (PhPIDs) to marketed medicinal products for human use. It is regularly updated as projects progress.
 Julia Nyman
Julia Nyman
September 2024: Global IDMP Working Group (GIDWG) yearly stakeholders’ meeting at Brazilian Health Regulatory Agency
Our past conferences have been full of valuable information and great networking opportunities. We expect this year to be the same. Welcome to our 4th Global IDMP Working Group (GIDWG) Stakeholder Meeting, planned for the third week of September 2024, hosted by the Brazilian Health Regulatory Agency (Anvisa) in Brazil.
UPDATE: Thank you to everyone who attended the 4th GIDWG Technical and Stakeholder Meeting for making it such a success. It provided an excellent platform to further our shared vision of global IDMP implementation.
Presentations materials:
End-to-end demonstration Q4 2023–Q2 2024
Testing of use cases for PhPID operating model
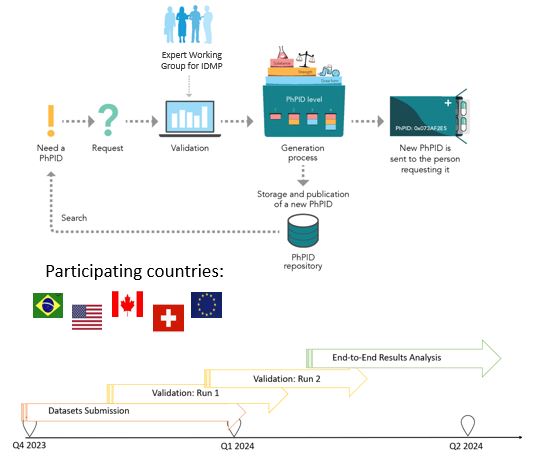
PURPOSE
To test the framework, including business rules, best practices, software, and operating model, for global IDMP implementation and maintenance of global identifiers for marketed products.
SCOPE
• Validate and generate PhPIDs for medicinal products based on GIDWG/EWG Business Rules
• EDQM and non-EDQM countries
• Similar products from different countries
• Larger batches and smaller data sets for regulators
• Test use cases for pharmacovigilance, drug shortages, and cross-border healthcare
STATUS
Completed
Global IDMP PhPID End-to-End Testing report, 5 December 2024
19–20 October: CTADHL sixth transatlantic workshop
This workshop will bring together experts involved in various aspects of IDMP implementation worldwide, from product development to regulatory processes, clinical use, and outcome assessments.
18 October: Global IDMP Working Group (GIDWG) stakeholders’ public meeting
Welcome to our 3rd Global IDMP Working Group (GIDWG) Stakeholder Meeting.
GIDWG’s mission is to establish a framework for global implementation of ISO IDMP standards and maintenance of global identifiers.
The GIDWG will report on 5 pilot projects:
- Substance Identifiers
- Dose form Identifiers
- Strength Definitions Identifiers
- Operational Model
- FHIR for information exchange.
These projects cover global use cases including pharmacovigilance, drug shortages, and cross-border healthcare.
Presentation materials:
