Testing of use cases for PhPID operating model
PURPOSE
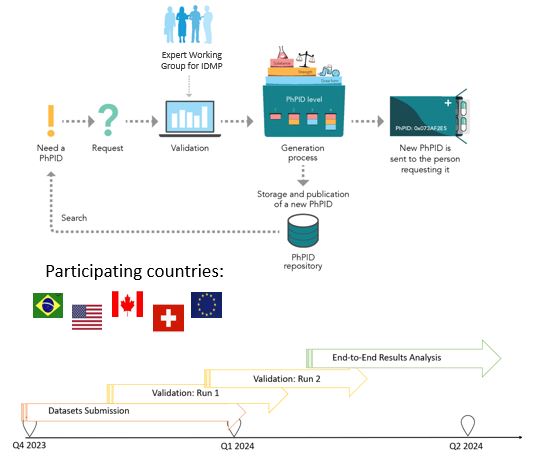
To test the framework, including business rules, best practices, software, and operating model, for global IDMP implementation and maintenance of global identifiers for marketed products.
SCOPE
• Validate and generate PhPIDs for medicinal products based on GIDWG/EWG Business Rules
• EDQM and non-EDQM countries
• Similar products from different countries
• Larger batches and smaller data sets for regulators
• Test use cases for pharmacovigilance, drug shortages, and cross-border healthcare
STATUS
Completed
Global IDMP PhPID End-to-End Testing report, 5 December 2024
